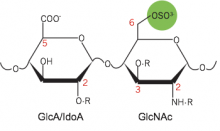
The prion-like spreading of tau aggregation is thought to drive progression of several neurodegenerative diseases, including Alzheimer’s Disease and other so-called “tauopathies”. The mechanisms by which tau aggregates spread between cells are unclear – but an understanding of the specific molecular players may provide us with potential therapeutic targets that inhibit disease progression. In collaboration with the groups of Dr. Ken Kosik (UC Santa Barbara) and Dr. Linda Hsieh-Wilson (Caltech), the Kampmann lab identified the molecules mediating the uptake of tau into cells: a specific type of sugar on cell surface proteins, 6-O-sulfated heparan sulfate proteoglycan. Dr. John Chen, a postdoctoral fellow in the Kampmann lab who is a QB3/Calico Longevity Fellow and the recipient of an Alzheimer’s Association postdoctoral fellowship, conducted a genetic screen for cellular tau uptake that identified the cellular enzymes generating this specific type of sugar. Stephanie See, a graduate student in the Kampmann lab and a recipient of the National Defense Science and Engineering Graduate Fellowship, generated the CRISPRi cell line in which the screen was conducted.
The results were published in the following article:
Rauch JN, Chen JJ, Sorum AW, Miller GM, Sharf T, See SK, Hsieh-Wilson LC, Kampmann M, Kosik KS (2018) Tau Internalization is Regulated by 6-O Sulfation on Heparan Sulfate Proteoglycans (HSPGs). Scientific Reports 8(1):6382. PMID: 29686391